The BiVACOR Total Artificial Heart Solution
Strategic Partner Specifications
A strategic partner of Calnetix Technologies, BiVACOR® is a clinical-stage medical device company pioneering long-term therapeutic solutions for patients with biventricular heart failure. The company is engaged in an extensive FDA-approved, first-in-human study to evaluate the safety and efficacy of the BiVACOR Total Artificial Heart (TAH).
Solution
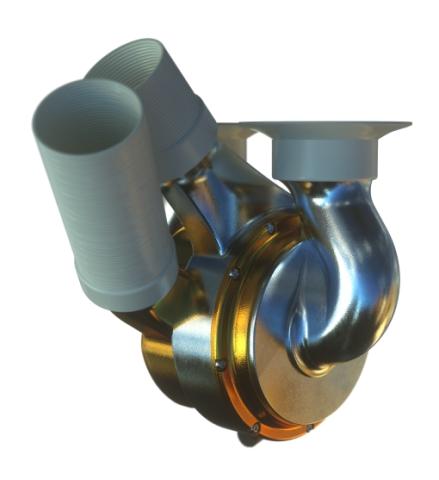
The BiVACOR TAH combines centrifugal rotary pump technology with Calnetix’s unique magnetic levitation (MAGLEV) bearing technology. The dual-side pump rotor is magnetically levitated between the two separate pump chambers and is the only moving component in the system. Unlike conventional mechanical bearings, this MAGLEV rotor suspension provides precise, stable operation without frictional wear and tear.
The MAGLEV bearings facilitate the movement of the pump’s double-sided centrifugal impeller, propelling blood from the respective pump chambers to the pulmonary (deoxygenated blood to the lungs) and systemic (oxygen-rich blood to tissue) circulations. These specially engineered pump impellers can produce enough cardiac output for an exercising adult male with relatively low power consumption. Additionally, the BiVACOR TAH features a patented flow-balancing system that permits dynamic adaptation to changes in the patient's hemodynamic physiology (e.g., blood pressure and cardiac output) by dynamically adjusting the precision control setpoint of the pump rotor using the MAGLEV bearings.
A small external smart controller drives pump operation, controls the MAGLEV system, and adapts to changes in patient activity and cardiac output demand. A compact, rechargeable battery system powers the TAH, supporting untethered functionality and greatly enhancing patient mobility. The BiVACOR TAH is constructed from titanium for maximum biocompatibility, corrosion resistance, and strength.
Challenges and Need
Heart failure is an increasingly prevalent global epidemic affecting at least 26 million people worldwide. For context, heart transplantations are reserved for those with severe heart failure and are limited to fewer than 6,000 procedures per year globally. BiVACOR is committed to addressing this global unmet need of patients with end-stage heart failure who are awaiting a transplant by innovating the next generation of life-extending technologies and solutions.
The BiVACOR team set out to conquer the limitations of current TAH technologies. Conventional TAH pumps feature volume displacement pumps and flexible polymer diaphragms to pump blood, whereas the BiVACOR TAH does not contain any of these components, maximizing durability and reliability. Unlike conventional TAH pumps, the BiVACOR TAH does not use a volume displacement pump or polymer diaphragms, improving durability and reliability.
Results
The First-in-Human implantation of the BiVACOR TAH was completed on July 9, 2024, at Baylor St. Luke’s Medical Center in the Texas Medical Center. The implantation represents an important milestone in the U.S. Food and Drug Administration (FDA) Early Feasibility Study (EFS) for the BiVACOR TAH.
The BiVACOR TAH is designed to be a long-term device that can replace the total function of the patient’s heart, representing a paradigm shift in artificial heart design. The small, compact device relies on proven rotary blood pump technology to reliably perform its life-sustaining functions.